Synthesis and characterization of heteropolymetallic complexes: thermally or photochemically induced reversible rearrangements.
Thermally reversible photochemical haptotropic rearrangement
Developing reversible intramolecular thermal and photochemical processes are interesting starting points for finding photofunctional molecules such as photochromic molecules and solar energy devices. We have recently reported that the haptotropic isomerization of diiron carbonyl complexes with an acenahthylene ligand, (Ace)Fe2(CO)5, is both thermally and photochemically induced in solution as well as the solid state. A photolysis/thermal treatment cycle of a KBr pellet with (1-A) or (1-B) results in a reversible interconversion between (1-A) and (1-B) in the solid state, which can be monitored by IR spectroscopy.
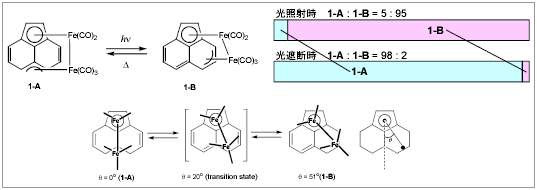
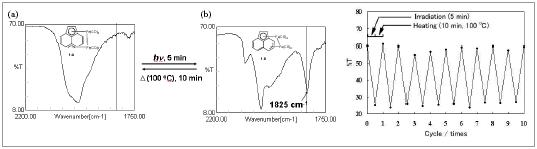
This cycle can be repeated over 10 times without changing the signal intensity. Thermally reversible photoisomerization of this compound is unique because the haptotropic rearrangement can potentially be used as photofunctional organometallic molecules.
Thermally or photochemically induced reductive cleavage of metal-metal bond
Photoirradiating a novel monomeric titanium complex, Cp2Ti(OtBu), which is synthesized from [Cp2TiCl]2 and KOtBu, with [CpRu(CO)2]2 affords a titanium-ruthenium dinuclear complex where the two metallic moieties are linked by a direct metal-metal bond. This reaction does not thermally proceed. An interesting feature of this Ti/Ru heterobimetallic complex is that the thermal fragmentation process can regenerate the starting materials, which is atypical because the formation of a Ti/Ru heterobimetallic complex is formally considered to be a metal-metal bond cleavage of ruthenium carbonyl dimers by a Ti(III) reducing reagent.
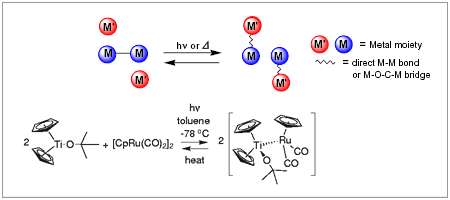
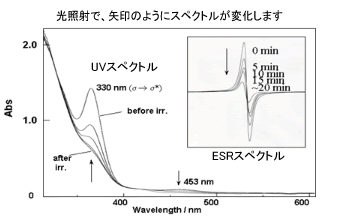
Because the starting titanium complex, Cp2Ti(OtBu), is paramagnetic and the obtained Ti/Ru heterobimetallic complex is diamagnetic, this reversible reaction changes the ESR spectrum as well as the color of the solution (see the Figure). Similar reactions of Cp2Ti(OtBu) with several transition metal carbonyl dimers also give rise to heterobimetallic complexes. Thus, heterobimetallic complexes are reversibly formed and are interesting organometallic molecules, which thermally or photochemically change the magnetic property due to the Ti(III) species. In this context, we are currently constructing a new type of reversible system.

