Self-encapsulation of homogeneous catalyst species into polymer gels: Access to a facile and efficient separation system for amine products in Ru-catalyzed reductions of carboxamides with polymethylhydrosiloxane (PMHS).
To protect our environment it is imperative to produce chemicals using environmentally benign processes. Thus, developing efficient and selective preparation methods using environmentally benign molecular catalysts have attracted great attention. Recently, we investigated the encapsulation of metal catalysts by polymers using a "self-encapsulation" system.
We have recently reported the reduction of amides using trialkylsilanes catalyzed by ruthenium cluster, (ā3,ā2,ā3,ā5-acenaphthylene)Ru3(CO)7 (cat 1). In the catalytic reduction of amides with polymer reducing reagents that contain Si-H groups such as polymethylhydrosiloxane (PMHS), ruthenium catalyst 1 produces the desired amines. At the same time, 1 forms a polymer gel from PMHS, which effectively self-encapsulates 1. After the reaction is complete, the amine product, which is soluble in the appropriate organic solvents, is facilely separated from the insoluble polymer gel, which contains the catalytic species. After the reaction, >99.95% of the ruthenium was encapsulated into the silicon resin and did not contaminate the amine product.

In addition, the present process can be applied to the reduction of various amides in high yields. The IR and 29Si-CPMAS analyses of the obtained polymer gel revealed that the triruthenium species may be immobilized to the cross-linking silicone resin via chemical bonding between the Ru and Si atoms and not by the physical encapsulation in the pore of the resin.
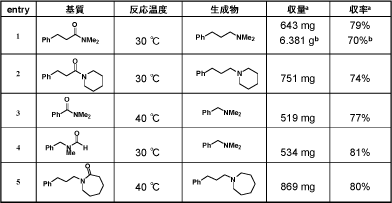
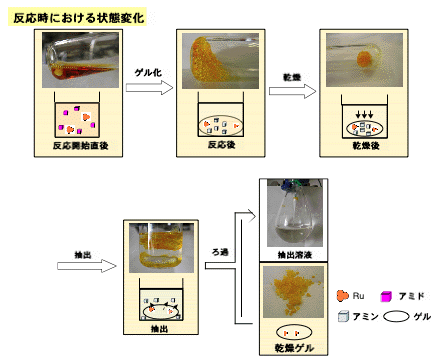
We believe that the present reduction of amides with PMHS is an environmental benign route for producing amines. Namely, it is an efficient catalytic process, which involves the facile separation of the metal and silicone byproducts from the product. This may be a good entry into a new field of environmentally benign chemical processes where the catalytic activation of polymer reagents leads to both an efficient chemical transformation of organic compounds, and removes metallic species and polymer byproducts from the desired products.

