Silane-induced reduction of organic substrates catalyzed by a triruthenium carbonyl cluster, (Ace)Ru3(CO)7.
Catalysis of organometallic clusters has received much attention due to its unique electronic state and its ability of gmultisite activationh toward organic substrates. In recent years, it has become increasingly important that certain clusters provide a novel class of homogeneous catalysts, which allow reactions that are unavailable by conventional mononuclear transition metal catalysts. We have focused on cluster catalysts with gflexibleh auxiliary ligands and their applications to catalytic reactions.
We prepared a triruthenium carbonyl cluster with an acenapthylene ligand in 1993, which readily reacts with molecular hydrogen or hydrosilanes to afford oxidative addition products. In other words, (Ace)Ru3(CO)7 effectively activates the H-H and Si-H bonds in this reaction.
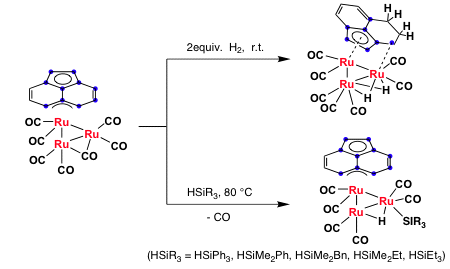
Scheme 1
We performed the hydrosililation (Scheme 1) of various organic substrates using (Ace)Ru3(CO)7 as the catalyst. Interestingly, (Ace)Ru3(CO)7 can effectively activate hydrosilane and can efficiently catalyze the reduction of various organic substrates such as ketones, esters, amides, etc.
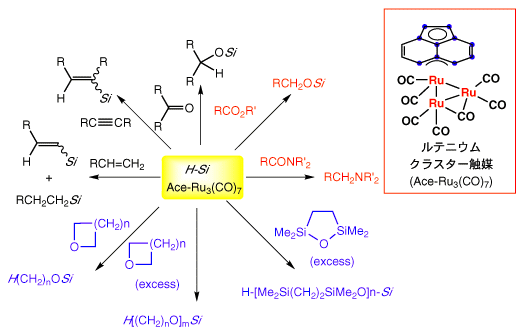
Scheme 2
Efficient catalytic hydrosililation (Scheme 2) of various organic substrates has been established using (Ace)Ru3(CO)7 as a cluster catalyst under mild conditions. The catalytic reduction of esters and amides are especially important because they do not readily undergo reductions using the standard method. Thus, ruthenium tricarbonyl cluster, (Ace)Ru3(CO)7, provides practical conditions for organic synthesis.

